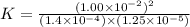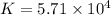Chemistry, 21.02.2020 19:18 carolelai08
At a particular temperature a 2.00-L flask at equilibrium contains 2.80 ✕ 10-4 mol N2, 2.50 ✕ 10-5 mol O2, and 2.00 ✕ 10-2 mol N2O. Calculate K at this temperature for the following reaction. 2 N2(g) + O2(g) equilibrium reaction arrow 2 N2O(g)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, shreyapatel2004
How many atoms of oxygen are contained in 160 grams of n2o3
Answers: 2

Chemistry, 22.06.2019 14:00, njones58emailtjcedu
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

You know the right answer?
At a particular temperature a 2.00-L flask at equilibrium contains 2.80 ✕ 10-4 mol N2, 2.50 ✕ 10-5 m...
Questions in other subjects:




Biology, 17.04.2021 23:00

Chemistry, 17.04.2021 23:00





Mathematics, 17.04.2021 23:00

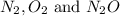
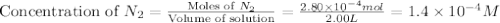

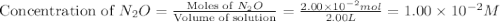
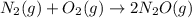
![K=\frac{[N_2O]^2}{[N_2][O_2]}](/tpl/images/0519/3945/0e61d.png)