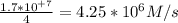Chemistry, 21.02.2020 18:30 naomirice24
The reaction between ethyl bromide (C₂H₅Br) and hydroxide ion in ethyl alcohol at 330 K, , is first order each in ethyl bromide and hydroxide ion. When [C₂H₅Br] is 0.0477 M and [OH⁻] is 0.100 M, the rate of disappearance of ethyl bromide is 1.7×10⁺⁷M/s.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, triddi666
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1



Chemistry, 22.06.2019 23:00, autumperry3599
What is the chemical formula for dihydrogen monoxide
Answers: 2
You know the right answer?
The reaction between ethyl bromide (C₂H₅Br) and hydroxide ion in ethyl alcohol at 330 K, , is first...
Questions in other subjects:

Mathematics, 20.10.2020 03:01

English, 20.10.2020 03:01


Mathematics, 20.10.2020 03:01

Mathematics, 20.10.2020 03:01


Mathematics, 20.10.2020 03:01



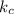 [A] where [A] is the concentration of the reagent
[A] where [A] is the concentration of the reagent![k_{c} \frac{[C_{2} H_{5} Br]}{2} \frac{[OH^{-} ]}{2}](/tpl/images/0519/3086/4c99a.png) =
= 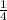 ×Kc[C₂H₅Br][OH⁻]
×Kc[C₂H₅Br][OH⁻]