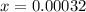Chemistry, 21.02.2020 18:35 michelle8978
Iodine and bromine react to give iodine monobromide, IBr. Ilg) + Br2(g) 2 Br(g) What is the equilibrium composition of a mixture at 145 C that initially contained 0.0019 mol each of iodine and bromine in a 5.0-L vessel? The equilibrium constant K, for this reaction at 145 C is 108.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, slugmilk1090
The agent of mechanical weathering in which rock is worn away by the grinding action of other rock particles is call
Answers: 1

Chemistry, 22.06.2019 00:30, tdowling331
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 22.06.2019 17:30, shookiegriffin
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1

Chemistry, 22.06.2019 19:20, halledoll2002
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3
You know the right answer?
Iodine and bromine react to give iodine monobromide, IBr. Ilg) + Br2(g) 2 Br(g) What is the equilibr...
Questions in other subjects:





Social Studies, 11.04.2021 22:40

Mathematics, 11.04.2021 22:40

Mathematics, 11.04.2021 22:40


Mathematics, 11.04.2021 22:40

Mathematics, 11.04.2021 22:40

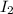 = 0.00006 M
= 0.00006 M 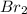 = 0.00006 M
= 0.00006 M 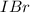 = 0.00064 M
= 0.00064 M 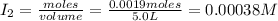
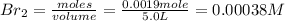
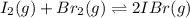
![K_c=\frac{[IBr]^2}{[I_2]\times [Br_2]}](/tpl/images/0519/3166/d47bc.png)
![108=\frac{[2x]^2}{(0.00038-x)^2}](/tpl/images/0519/3166/891f8.png)