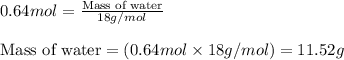Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:30, dimondqueen511
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 08:00, ggdvj9gggsc
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1
You know the right answer?
Trisilane (Si3H8) is a liquid with a density of 0.739 g cm-3. It reacts with oxygen to give silicon...
Questions in other subjects:


Mathematics, 09.11.2020 20:50

Mathematics, 09.11.2020 20:50


Mathematics, 09.11.2020 20:50

Physics, 09.11.2020 20:50



Arts, 09.11.2020 20:50

Spanish, 09.11.2020 20:50

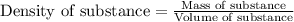
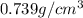
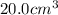
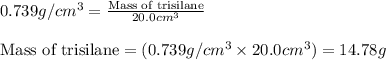
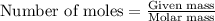 .....(1)
.....(1)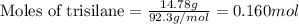
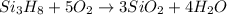
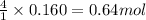 of water
of water