Chemistry, 21.02.2020 05:58 skylarschumacher7
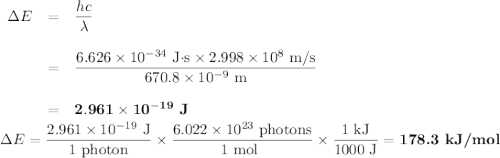
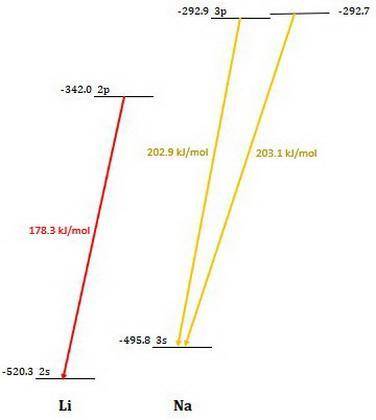
Calculate the photon energy (in kJ/mol) for the single Li emission and the two Na emission wavelengths. To accomplish this, first calculate the energy in units of /photon from Equation (4) and then multiply the result by Avogadro's number to express the energy in /mol of photons. Lastly, divide by 1000 to convert this result to units of kl/mol. Next, use the photon energies to determine the valence orbital energies for both Li and Na. For lithium, the transition is from the 2p- to the 2s-orbital, and the 2s-orbital energy is -520.3 kJ/mol. Use this to find the energy of the 2p-orbitals in Li. For sodium, the higher-energy photon is emitted when the electron drops from one of the 3p-orbitals to the 3s-orbital, while the lower energy photon is emitted when the electron drops from one of the 3d-orbitals to one of the 3p-orbitals. Use these facts, along with the known energy of the 3s-orbital (-495.8 kJ/mol), to find the energies of the 3p- and 3d-orbitals.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, kingteron5870
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1


Chemistry, 22.06.2019 10:00, nana54muller
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 13:30, yasiroarafat12
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1
You know the right answer?
Calculate the photon energy (in kJ/mol) for the single Li emission and the two Na emission wavelengt...
Questions in other subjects:


Physics, 22.09.2021 04:30

Mathematics, 22.09.2021 04:30

Law, 22.09.2021 04:30


Mathematics, 22.09.2021 04:30

Mathematics, 22.09.2021 04:30

Mathematics, 22.09.2021 04:30

Biology, 22.09.2021 04:30