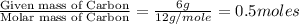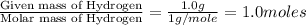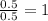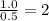Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:10, Tyrant4life
Which of these is the result of scientific research and not engineering? a. a new shoe design that features air cushioning for more comfort and protection b. the creation of glass with uv protection. c. a conclusion about diet commonalities among diabetics. d. the development of a smaller, more compact missile.
Answers: 1

Chemistry, 22.06.2019 06:00, Sarahinator04
0.09 moles of sodium sulfate in 12 ml of solution
Answers: 3

You know the right answer?
A 7.0 g sample of a hydrocarbon (a molecule that has only hydrogen and carbon) is subject to combust...
Questions in other subjects:





Mathematics, 26.04.2020 03:19


History, 26.04.2020 03:19


Geography, 26.04.2020 03:19

Engineering, 26.04.2020 03:19

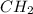
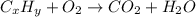
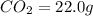
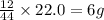 of carbon will be contained.
of carbon will be contained.