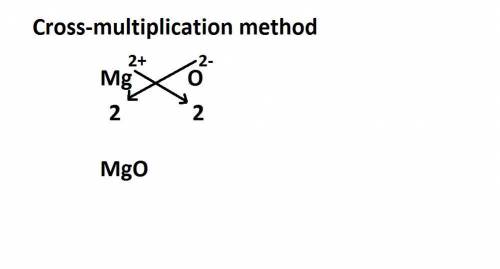
"Atoms of magnesium and oxygen form ions and ionic bonds "The difference is that instead of having each atom gain or lose one electron, each atom of one of these elements loses two electrons, and each atom of the other element gains two electrons. Describe the process that leads to the formation of the ionic bond between magnesium and oxygen atoms in magnesium oxide.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:50, kyleighmarie05
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3

Chemistry, 22.06.2019 00:10, scottbrandon653
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 03:40, jude3412
In an effort to address concerns about global warming, a power plant in portland, oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1
You know the right answer?
"Atoms of magnesium and oxygen form ions and ionic bonds "The difference is that instead of having e...
Questions in other subjects:

Mathematics, 16.09.2020 18:01

Mathematics, 16.09.2020 18:01

Mathematics, 16.09.2020 18:01

Mathematics, 16.09.2020 18:01

Mathematics, 16.09.2020 18:01

Mathematics, 16.09.2020 18:01

Mathematics, 16.09.2020 18:01

Mathematics, 16.09.2020 18:01

Mathematics, 16.09.2020 18:01

Social Studies, 16.09.2020 18:01

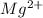 ions.
ions.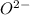 ions.
ions. 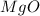 ).
).