
Chemistry, 21.02.2020 05:09 ilovepickles930
3.00 mol of an ideal gas with CV, m=3R/2 undergoes an expansion from an initial state described by T=310.K and P=6.00bar against a constant external pressure of zero bar until the final pressure is equal to one-fifth of its initial value. The state of the surroundings is T=298K, P=0.250bar. a. FInd entropy of surroundings.
b. Find total entropy

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, tylerineedhelp
Ihat will happen if i added baking soda to vinegar
Answers: 2


Chemistry, 22.06.2019 11:00, snowprincess99447
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

You know the right answer?
3.00 mol of an ideal gas with CV, m=3R/2 undergoes an expansion from an initial state described by T...
Questions in other subjects:





Mathematics, 19.11.2020 19:00


Mathematics, 19.11.2020 19:00



English, 19.11.2020 19:00

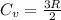
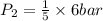 > \frac{1}{5} \times P_{1}[/tex]
> \frac{1}{5} \times P_{1}[/tex]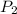 = 1.2 bar
= 1.2 bar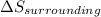 = 0
= 0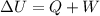
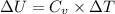
 = 0 this means that
= 0 this means that 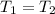
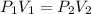
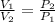
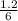
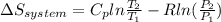
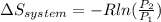
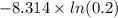

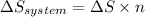
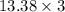
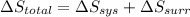 > 0
> 0

