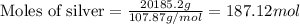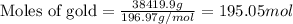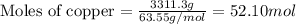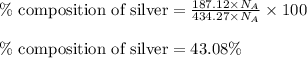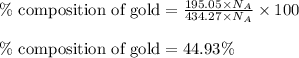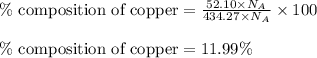Chemistry, 21.02.2020 03:55 angelreji4702
What is the composition, in atom percent, of an alloy that contains a) 44.5 lbm of silver, b) 84.7 lbm of gold, and c) 7.3 lbm of Cu? The atomic weights for silver, gold, and copper are, respectively, 107.87, 196.97, and 63.55 g/mol.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, joelpimentel
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 08:40, kellymcdow5135
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 10:30, zayam1626
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 12:20, sindy35111
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l. s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3
You know the right answer?
What is the composition, in atom percent, of an alloy that contains a) 44.5 lbm of silver, b) 84.7 l...
Questions in other subjects:

Mathematics, 16.01.2020 05:31


Mathematics, 16.01.2020 05:31

Law, 16.01.2020 05:31





Computers and Technology, 16.01.2020 05:31

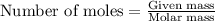 ......(1)
......(1)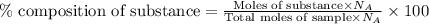 ......(2)
......(2)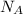 = Avogadro's number
= Avogadro's number