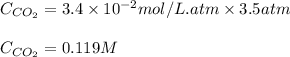Chemistry, 21.02.2020 03:00 Brennen435
The partial pressure of carbon dioxide in a gas mixture is 3.5 atm. What will be the solubility (in M) of carbon dioxide gas when Henry's Law constant for carbon dioxide is 3.4 x 10-2M/atm

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, shadekashakay
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 04:00, speris1443
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1


Chemistry, 22.06.2019 16:40, roderickhinton
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3
You know the right answer?
The partial pressure of carbon dioxide in a gas mixture is 3.5 atm. What will be the solubility (in...
Questions in other subjects:

History, 19.09.2021 01:00

History, 19.09.2021 01:00

Mathematics, 19.09.2021 01:00



Chemistry, 19.09.2021 01:00

Mathematics, 19.09.2021 01:00

History, 19.09.2021 01:00

Mathematics, 19.09.2021 01:00

Mathematics, 19.09.2021 01:00

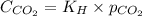
 = Henry's constant =
= Henry's constant = 
 = partial pressure of carbon dioxide gas = 3.5 atm
= partial pressure of carbon dioxide gas = 3.5 atm