
Chemistry, 21.02.2020 03:00 darenl4478
The Fe 2 + ( 55.845 g/mol) content of a 2.349 g steel sample dissolved in 50.00 mL of an acidic solution was determined by tiration with a standardized 0.100 M potassium permanganate ( KMnO 4 , 158.034 g/mol) solution. The titration required 43.16 mL to reach the end point. What is the concentration of iron in the steel sample? Express your answer as grams of Fe per gram of steel. MnO − 4 + 8 H + + 5 Fe 2 + − ⇀ ↽ − Mn 2 + + 5 Fe 3 + + 4 H 2 O

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, maddynichole2017
Read these sentences from the text. near the equator, the tropics receive the most rain on a consistent basis. as a result, the fresh water falling into the ocean decrease the salinity of the surface water in that region. [. .] . . as the salt content of sea water increases, so does its density. what can you infer about how rain affects the density of surface water near the equator?
Answers: 1

Chemistry, 22.06.2019 01:30, adrian08022
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2

Chemistry, 22.06.2019 05:30, ayoismeisjjjjuan
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 23.06.2019 03:50, KAITLYN007
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer.
Answers: 1
You know the right answer?
The Fe 2 + ( 55.845 g/mol) content of a 2.349 g steel sample dissolved in 50.00 mL of an acidic solu...
Questions in other subjects:


Mathematics, 06.05.2020 22:03


Mathematics, 06.05.2020 22:03

Biology, 06.05.2020 22:03

Mathematics, 06.05.2020 22:03





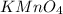 solution = 0.100 M
solution = 0.100 M
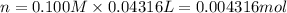
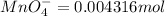
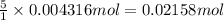 of ferrous ions
of ferrous ions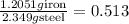 iron per gram of steel
iron per gram of steel

