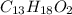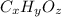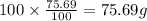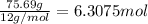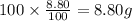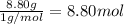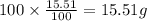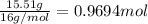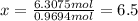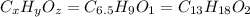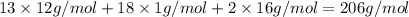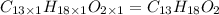Chemistry, 21.02.2020 02:26 sergun252005
Determine the empirical and molecular formulas of each of the following substances. For example, butane has an empirical formula of C2H5 (lowest whole-number ratio) and a molecular formula of C4H10, where the molecular formula corresponds to the molar mass of 58.12 g/mol.
Ibuprofen, a headache remedy, contains 75.69% C, 8.80% H, and 15.51% O by mass and has a molar mass of 206 g/mol.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, WinterStrikesBack
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 12:20, tenleywood
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 17:00, destinyycooper
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2
You know the right answer?
Determine the empirical and molecular formulas of each of the following substances. For example, but...
Questions in other subjects:

Mathematics, 26.04.2020 06:39




Biology, 26.04.2020 06:39

Mathematics, 26.04.2020 06:39



Mathematics, 26.04.2020 06:39