
Chemistry, 21.02.2020 02:27 nayelimoormann
Consider the following equilibrium process at 686°C. CO2(g) + H2(g) equilibrium reaction arrow CO(g) + H2O(g) The equilibrium concentrations of the reacting species are [CO] = 0.050 M, [H2] = 0.045 M, [CO2] = 0.086 M, and [H2O] = 0.040 M. (a) Calculate Kc for the reaction at 686°C. WebAssign will check your answer for the correct number of significant figures. (b) If we add CO2 to increase its concentration to 0.50 mol/L, what will the concentrations of all the gases be when equilibrium is reestablished?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 19:00, georgesarkes12
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2


Chemistry, 23.06.2019 02:20, alejandraluna95
Why dose heating increase the speed at which a solution dissolved in water
Answers: 1
You know the right answer?
Consider the following equilibrium process at 686°C. CO2(g) + H2(g) equilibrium reaction arrow CO(g)...
Questions in other subjects:

Biology, 20.10.2019 11:30

Mathematics, 20.10.2019 11:30


History, 20.10.2019 11:30



Mathematics, 20.10.2019 11:30

Business, 20.10.2019 11:30


Social Studies, 20.10.2019 11:30

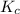 is 0.52.
is 0.52.![[CO_2] = 0.4748 M](/tpl/images/0518/6100/1098f.png)
![[H_2] = 0.0198 M](/tpl/images/0518/6100/4696c.png)
![[CO] = 0.0752 M](/tpl/images/0518/6100/1d075.png)
![[H_2O] =0.0652 M](/tpl/images/0518/6100/30296.png)
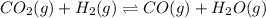
![[CO] = 0.050 M, [H_2] = 0.045 M, [CO_2] = 0.086 M, and [H_2O] = 0.040 M](/tpl/images/0518/6100/10b47.png)
![\K_c=\frac{[CO][H_2O]}{[CO_2][H_2]}](/tpl/images/0518/6100/2fcaf.png)
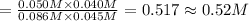
![K_c=\frac{[CO][H_2O]}{[CO_2][H_2]}](/tpl/images/0518/6100/c597d.png)
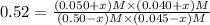
![[CO_2] = (0.50-x) M=(0.50-0.0252)M = 0.4748 M](/tpl/images/0518/6100/7357e.png)
![[H_2] = (0.045-x) M= (0.045-0.0252) M=0.0198 M](/tpl/images/0518/6100/31260.png)
![[CO] = (0.050+x) M=(0.050+0.0252)M = 0.0752 M](/tpl/images/0518/6100/0b9e4.png)
![[H_2O] = (0.040+x) M=(0.040+0.0252) M=0.0652 M](/tpl/images/0518/6100/b7e5e.png)


