Chemistry, 21.02.2020 01:24 INEEDTOPASSS
Determine the balanced chemical equation for this reaction. C8H18(g)+O2(g)→CO2(g)+H2O(g)
Part A:
Enter the coefficients for each compound in order, separated by commas. For example, 1,2,3,4 would indicate one mole of C8H18, two moles of O2, three moles of CO2, and four moles of H2O.
2,25,16,18
Part B:
0.280 mol of octane is allowed to react with 0.630 mol of oxygen. Which is the limiting reactant?
Oxygen
Part C:
How many moles of water are produced in this reaction?
H2O produced = 0.454 mol
Part D:
After the reaction, how much octane is left?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:00, maymayrod2000
A32 year old immigrant from a patriarchal country is giving birth. as she is delivering the baby, she tearfully confesses to her doctor that this is her 4th child and she simply cannot handle any more children. she tells the doctor that her husband refuses to use contraception or allow her to, and she begs her doctor to tie her tubes and not tell her husband. the doctor complies. was hipaa violated? why or why not?
Answers: 3


Chemistry, 22.06.2019 08:30, waterborn7152
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2

Chemistry, 22.06.2019 11:50, robert7248
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3
You know the right answer?
Determine the balanced chemical equation for this reaction. C8H18(g)+O2(g)→CO2(g)+H2O(g)
...
...
Questions in other subjects:


History, 23.06.2021 17:50

Biology, 23.06.2021 17:50

English, 23.06.2021 17:50

Chemistry, 23.06.2021 17:50



History, 23.06.2021 17:50


Mathematics, 23.06.2021 17:50

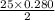 moles of oxygen
moles of oxygen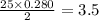
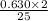 moles of octane will react with the 0.630 mole of oxygen present
moles of octane will react with the 0.630 mole of oxygen present