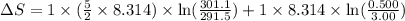Chemistry, 21.02.2020 01:05 AsiaDeas4078
During the test of an internal combustion engine, 3.00 L of nitrogen gas at 18.5 °C was compressed suddenly (and irrevers- ibly) to 0.500 L by driving in a piston. In the process, the tempera- ture of the gas increased to 28.1°C. Assume ideal behavior, what is the change in entropy of the gas?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, ellaemtagedeane
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 15:30, lizzyhearts
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 22.06.2019 20:00, Isaiahtate053
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3

Chemistry, 23.06.2019 11:30, elpeke102p73fz3
Jenny places a strip of ph paper into a solution. when she removes the ph paper, it has turned yellow-green. what should jenny do next to determine the ph of her solution? a. use a different testing method because the ph paper should not change colors b. place the ph paper into a machine that reads the ph of the solution c. compare the ph paper's color with the color of ph paper from another solution d. compare the ph paper's color with a chart of colors and ph ranges
Answers: 1
You know the right answer?
During the test of an internal combustion engine, 3.00 L of nitrogen gas at 18.5 °C was compressed s...
Questions in other subjects:





Chemistry, 16.04.2020 23:06

Geography, 16.04.2020 23:06

Mathematics, 16.04.2020 23:07


Mathematics, 16.04.2020 23:07

English, 16.04.2020 23:07

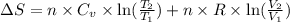
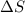 = change in molar entropy
= change in molar entropy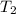 = final temperature =
= final temperature = 
 = initial temperature =
= initial temperature = 
 = final volume = 0.500 L
= final volume = 0.500 L = initial volume = 3.00 L
= initial volume = 3.00 L = heat capacity diatomic gas
= heat capacity diatomic gas  =
=