
Chemistry, 21.02.2020 01:05 lucifer6669
Determine the moles of H+ reacting with the metal based on the following experimental data. 10.00 mL of 1.00 M HCl solution is added to a sample of Mg metal. The reaction goes to completion and all of the Mg metal is gone. 27.08 mL of 0.30 M NaOH solution is required to reach the end point when titrating the remaining HCl.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:00, applejulianamoreno
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3


Chemistry, 23.06.2019 11:30, elpeke102p73fz3
Jenny places a strip of ph paper into a solution. when she removes the ph paper, it has turned yellow-green. what should jenny do next to determine the ph of her solution? a. use a different testing method because the ph paper should not change colors b. place the ph paper into a machine that reads the ph of the solution c. compare the ph paper's color with the color of ph paper from another solution d. compare the ph paper's color with a chart of colors and ph ranges
Answers: 1
You know the right answer?
Determine the moles of H+ reacting with the metal based on the following experimental data. 10.00 mL...
Questions in other subjects:

Mathematics, 23.02.2021 09:40


History, 23.02.2021 09:40

Mathematics, 23.02.2021 09:40

English, 23.02.2021 09:40

Mathematics, 23.02.2021 09:40


Chemistry, 23.02.2021 09:40


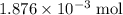 .
. .
.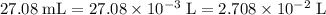 .
.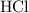 initially present (in the
initially present (in the 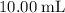 solution at
solution at 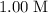 .)
.)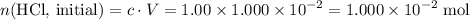 .
.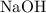 from the titration:
from the titration: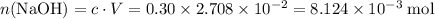 .
.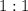 ratio:
ratio: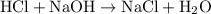 .
.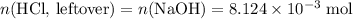 .
.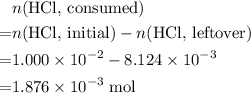 .
.

