Chemistry, 20.02.2020 23:42 xxleeciexx
Use the ΔHrxn values of the following reactions: 2SO2(g) + O2(g) → 2SO3(g) ΔHrxn = –196 kJ 2S(s) + 3O2(g) → 2SO3(g) ΔHrxn = –790 kJ to calculate the ΔHrxn value of this reaction: S(s) + O2(g) → SO2(g) ΔHrxn = ?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, sophiebeardsley94
Aaspirin has a density of 1.40 g/cm^3 what is the volume in cubic centimeters of a tablet weighing 320 mg?
Answers: 3

Chemistry, 22.06.2019 12:50, martinez6221
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 15:20, shanyeah
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1
You know the right answer?
Use the ΔHrxn values of the following reactions: 2SO2(g) + O2(g) → 2SO3(g) ΔHrxn = –196 kJ 2S(s) + 3...
Questions in other subjects:

Mathematics, 21.05.2021 01:30


Mathematics, 21.05.2021 01:30



English, 21.05.2021 01:30

Mathematics, 21.05.2021 01:30



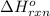 for the reaction is -297 kJ.
for the reaction is -297 kJ.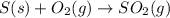
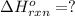
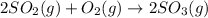
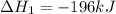
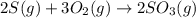
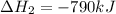
![\Delta H^o_{rxn}=\frac{[1\times (-\Delta H_1)]+[1\times \Delta H_2]}{2}](/tpl/images/0518/2732/98145.png)