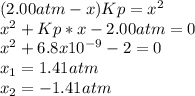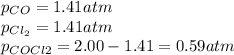Chemistry, 20.02.2020 23:05 transfergiecek8765
Lexan is a plastic used to make compact discs, eyeglass lenses, and bullet-proof glass. One of the compounds used to make Lexan is phosgene (COCl2), an extremely poisonous gas. Phosgene decomposes by the following reaction for which Kp = 6.8 ✕ 10-9 at 100°C. COCl2(g) equilibrium reaction arrow CO(g) + Cl2(g) If pure phosgene at an initial pressure of 2.0 atm decomposes, calculate the equilibrium pressures of all species.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 06:30, destineedeal1
What type of chemical reaction occurs between silver nitrate (agno3) and copper (cu)? the equation i was given is 2agno3 + cu —> 2ag+ cu(no3)2.
Answers: 1

Chemistry, 23.06.2019 08:00, mantha0402
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 1

Chemistry, 23.06.2019 11:50, halllawson
What is the oxidation half-reaction for this unbalanced redox equation? cr2o72– + fe2+ → cr3+ + fe3+ cr3+ → cr2o72– cr2o72– → cr3+ fe3+ → fe2+ fe2+ → fe3+?
Answers: 2

You know the right answer?
Lexan is a plastic used to make compact discs, eyeglass lenses, and bullet-proof glass. One of the c...
Questions in other subjects:

Mathematics, 22.04.2021 14:00

Chemistry, 22.04.2021 14:00

Mathematics, 22.04.2021 14:00


Computers and Technology, 22.04.2021 14:00

History, 22.04.2021 14:00

Health, 22.04.2021 14:00

Mathematics, 22.04.2021 14:00


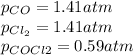
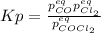
 , the law of mass action becomes:
, the law of mass action becomes: