
Chemistry, 20.02.2020 22:41 cmflores3245
What is the vapor pressure of the solution if 35.0 g of water is dissolved in 100.0 g of ethyl alcohol at 25 ∘C? The vapor pressure of pure water is 23.8 mmHg, and the vapor pressure of ethyl alcohol is 61.2 mmHg at 25 ∘C.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 23:00, soccerplayer17
What is the number of neutrons in an atom with atomic mass of 35
Answers: 2

Chemistry, 23.06.2019 03:30, antoinetteee03
Name atleast 3 type of energy associated with the microwave
Answers: 1
You know the right answer?
What is the vapor pressure of the solution if 35.0 g of water is dissolved in 100.0 g of ethyl alcoh...
Questions in other subjects:

Mathematics, 03.02.2022 02:00

Mathematics, 03.02.2022 02:00


Mathematics, 03.02.2022 02:00


History, 03.02.2022 02:00


Mathematics, 03.02.2022 02:00

History, 03.02.2022 02:00

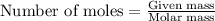 .....(1)
.....(1)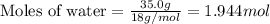
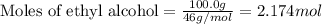
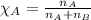
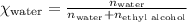
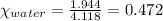
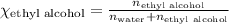
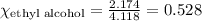
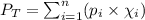
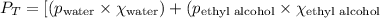
![p_T=[(23.8\times 0.472)+(61.2\times 0.528)]\\\\p_T=43.55mmHg](/tpl/images/0518/1070/83e05.png)


