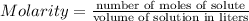Chemistry, 20.02.2020 22:27 mbrisen7420
You make 1.000 L of an aqueous solution that contains 35.0 g of ribose (C5H10O5). How many liters of water would you have to add to this solution to reduce the molarity you calculated in Part A (.233 moles) by a factor of two?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:30, jocelynmarquillo1
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 17:00, brownvester44
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 23.06.2019 01:00, zitterkoph
Which of the following is a physical change? a. burning a piece of wood b. sawing a piece of wood in half c. rust forming on an iron fence d. a copper roof changing color from orange to green
Answers: 1
You know the right answer?
You make 1.000 L of an aqueous solution that contains 35.0 g of ribose (C5H10O5). How many liters of...
Questions in other subjects:

Chemistry, 08.04.2021 18:40

Mathematics, 08.04.2021 18:40

History, 08.04.2021 18:40

English, 08.04.2021 18:40




Biology, 08.04.2021 18:40

Mathematics, 08.04.2021 18:40

Mathematics, 08.04.2021 18:40