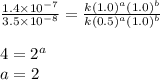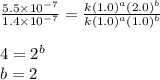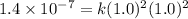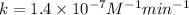Chemistry, 20.02.2020 17:19 annafellows
1. What is the rate law and the value of the rate constant for the reaction: 2 AB2 + C -- CB What is the order for each reactant and the overall order for the reaction. Write correct label for ""k"" rate constant. Trail [AB2] [C] Rate of CB 1 1.0 1.0 1.4*10-7 M/min 2 0.5 1.0 3.5*10-8 M/min 3 1.0 2.0 5.5*10-7 M/min

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 22:30, darkshaders11
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1

Chemistry, 22.06.2019 23:30, treylartigue
The appropriate concentration for an iodine sanitizer is
Answers: 1

Chemistry, 23.06.2019 01:30, jarteria0
Some molecular compounds, such as hcl, ionize completely in solution. for molecular compounds such as h2co3, most molecules do not ionize in solution. which describes the properties of these two solutes? a. hcl and h2co3 have the same effect on the properties of the solution. b. hcl raises the freezing point of water more than h2co3 does. c. hcl raises the boiling point of water more than h2co3 does.
Answers: 2
You know the right answer?
1. What is the rate law and the value of the rate constant for the reaction: 2 AB2 + C -- CB What i...
Questions in other subjects:


Health, 29.09.2019 14:30

History, 29.09.2019 14:30

History, 29.09.2019 14:30


Mathematics, 29.09.2019 14:30

History, 29.09.2019 14:30



Spanish, 29.09.2019 14:30

![\text{Rate}=k[AB_2]^2[C]^2](/tpl/images/0517/4966/e3c4b.png)
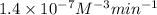
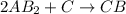
![\text{Rate}=k[AB_2]^a[C]^b](/tpl/images/0517/4966/89897.png)
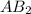
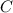
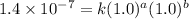 ....(1)
....(1)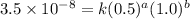 ....(2)
....(2)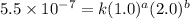 ....(3)
....(3)