Chemistry, 20.02.2020 15:19 lionessny6301
All the squares outlined on the model cells are 1 cm by 1 cm.
.Each face of Cube A measures 1 cm long by 1 cm wide.
.Each face of Cube B measures 2 cm long by 2 cm wide.
.Each face of Cube C measures 3 cm long by 3 cm wide.
.Calculate the area of one side of each cube by multiplying the length times the width.
.Calculate the surface area of each cube by multiplying the area of one side of the cube by 6 sides.
.Calculate the volume of each cube by multiplying length times width times height.
.Build the ratio for each cube: (surface area)/volume
.For each cube, divide surface area by volume and round off to the nearest whole number. For example, 1.5 to 1 is 3:2.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, breannaasmith1122
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1



Chemistry, 23.06.2019 15:30, expeditionofsin
How many moles of potassium nitrate, kno3 are present in a sample with a mass of 85.2 g?
Answers: 1
You know the right answer?
All the squares outlined on the model cells are 1 cm by 1 cm.
.Each face of Cube A measu...
.Each face of Cube A measu...
Questions in other subjects:


Mathematics, 03.02.2021 23:10




Health, 03.02.2021 23:10

History, 03.02.2021 23:10

Mathematics, 03.02.2021 23:10

Mathematics, 03.02.2021 23:10

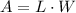
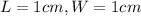
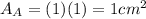
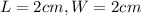
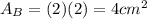
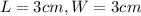
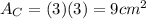
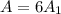
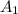 is the area of one face of the cube
is the area of one face of the cube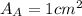
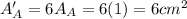
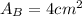
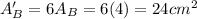
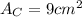
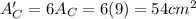
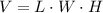
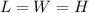
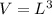
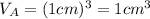
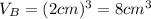
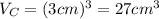
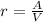
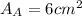 (surface area)
(surface area)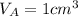 (volume)
(volume)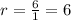
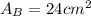 (surface area)
(surface area)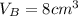 (volume)
(volume)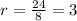
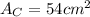 (surface area)
(surface area)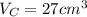 (volume)
(volume)