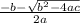Chemistry, 20.02.2020 08:37 thestuckone
At 700.°C, the total pressure of the system is found to be 3.01 atm. If the equilibrium constant KP is 1.52, calculate the equilibrium partial pressures of CO2 and CO.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, aedmund1225
Agas has a volume of 0.7 l at 300 mmhg. what would be the new volume at 900 mmhg
Answers: 1

Chemistry, 22.06.2019 07:30, deidaraXneji
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 22.06.2019 13:00, nadiarose6345
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 22.06.2019 21:30, kawaiiblurainbow
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1
You know the right answer?
At 700.°C, the total pressure of the system is found to be 3.01 atm. If the equilibrium constant KP...
Questions in other subjects:


Social Studies, 22.02.2021 22:30

History, 22.02.2021 22:30


History, 22.02.2021 22:30



Mathematics, 22.02.2021 22:30


 = 1.5 atm
= 1.5 atm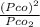
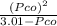 ⇒ 1.52 =
⇒ 1.52 = 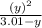 =
= 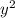 = 1.52 (3.01 - y)
= 1.52 (3.01 - y) 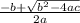 and y =
and y =