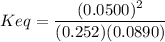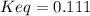Chemistry, 20.02.2020 06:15 aliyahmuhammad5197
An aqueous mixture of phenol and ammonia has initial concentrations of 0.302 M C6H5OH(aq) and 0.139 M NH3(aq). At equilibrium, the C6H5O–(aq) concentration is 5.00E-2 M. Calculate K for the reaction.

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 12:30, meghan2529
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1
You know the right answer?
An aqueous mixture of phenol and ammonia has initial concentrations of 0.302 M C6H5OH(aq) and 0.139...
Questions in other subjects:




Computers and Technology, 20.09.2019 05:30


Mathematics, 20.09.2019 05:30

Mathematics, 20.09.2019 05:30

Mathematics, 20.09.2019 05:30




![Keq=\dfrac{[C_6H_5OH(aq)].[NH_{3}(aq)]}{[C_6H_5O^-(aq)].[NH_4^+(aq)]}](/tpl/images/0517/0091/ca7b5.png)