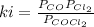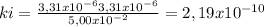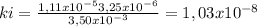Chemistry, 20.02.2020 05:59 kayranicole1
At 100oC the equilibrium constant for the reaction below has the value of Keq = 2.19 x 10−10. COCl2(g) ⇄ CO(g) + Cl2(g) Are the following mixtures of COCl2, CO, and Cl2 at equilibrium? If not, indicate the direction that the reaction must proceed to achieve equilibrium. (i) PCOCl2 = 5.00 × 10−2 atm; PCO = 3.31 × 10−6 atm; PCl2 = 3.31 × 10−6 atm (ii) PCOCl2 = 3.50 × 10−3 atm; PCO = 1.11 × 10−5 atm; PCl2= 3.25 × 10−6 atm
(i) not at equilibrium, left to right (ii) equilibrium
(i) equilibrium, (ii) not at equilibrium, right to left
(i) equilibrium, (ii) not at equilibrium, left to right
(i) not at equilibrium, right to left, (ii) equilibrium
(i) equilibrium, (ii) equilibrium

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:30, haileywebb8
If you want to create an electrical current, which situation would produce a solution capable of this
Answers: 3

Chemistry, 21.06.2019 23:00, orlando19882000
Ahypothrticalax type of ceramic material is known to have a density of 2.10 g/cm3 and a unit cell of cubic symmetry with a cell edge length of 0.57 nm. the atomic weights of the a and x elements are 28.5and 30.0 g/mol, respectively. on the basis of this information, which of the following crystal structures is (are) possible for this material: sodium chloride, cesium chloride, or zinc blende
Answers: 1

Chemistry, 22.06.2019 13:00, nauticatyson9
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1
You know the right answer?
At 100oC the equilibrium constant for the reaction below has the value of Keq = 2.19 x 10−10. COCl2(...
Questions in other subjects:




Mathematics, 31.08.2019 00:00


History, 31.08.2019 00:00


Mathematics, 31.08.2019 00:00

Biology, 31.08.2019 00:00

Mathematics, 31.08.2019 00:00