
Ethanol (C2H5OH) melts a - 144 oC and boils at 78 °C. The enthalpy of fusion of ethanol is 5.02 kj/mol, and its enthalpy of vaporization is 38.56 kj/mol. The specific heats of solid and liquid ethanol are 0.97j/g - k and 2.3 j/g - K, respectively.
(a) How much heat is required to convert 42.0 g of ethanol at 35 °C to the vapor phase at 78 °C?
(b) How much heat is required to convert the same amount of ethanol at - 155 oC to the vapor phase at 78 °C?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:20, carcon2019
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3

Chemistry, 23.06.2019 00:30, vane6176
You are attempting to recrystallize a crude product mixture. you add the appropriate amount of hot solvent and are allowing the solution to slowly cool to room temperature. however, at room temperature no crystals have appeared, which of the following methods should be used to induce crystallization? choose all correct answers. a) place the flask in an ice bath. b) swirl the contents of the flask. c) add a small seed crystal of the desired product. d) scratch the inside of the glassware using a stir rod. it can be multiple choices
Answers: 3

Chemistry, 23.06.2019 10:00, tammydbrooks43
What is the density, d, of a substance with a volume of v = 12.5 cm3 and a mass of m = 74.4 g ?
Answers: 1
You know the right answer?
Ethanol (C2H5OH) melts a - 144 oC and boils at 78 °C. The enthalpy of fusion of ethanol is 5.02 kj/m...
Questions in other subjects:



Biology, 27.10.2020 07:30


Mathematics, 27.10.2020 07:30


Chemistry, 27.10.2020 07:30

Mathematics, 27.10.2020 07:40


History, 27.10.2020 07:40

 .........(1)
.........(1) = specific heat capacity of substance
= specific heat capacity of substance = change in temperature
= change in temperature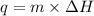 ........(2)
........(2)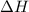 = enthalpy of the reaction
= enthalpy of the reaction
![m=42.0g\\c_l=2.3J/g.K\\T_2=78^oC\\T_1=35^oC\\\Delta T=[T_2-T_1]=[78-35]^oC=43^oC=43K](/tpl/images/0516/7715/feb03.png)
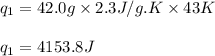


![[q_1+q_2]](/tpl/images/0516/7715/7bba2.png)
![[4153.8J+32467.7J]=36621.5J](/tpl/images/0516/7715/59544.png)

![m=42.0g\\c_s=0.97J/g.K\\T_2=-144^oC\\T_1=-155^oC\\\Delta T=[T_2-T_1]=[-144-(-155)]^oC=11^oC=11K](/tpl/images/0516/7715/d4732.png)
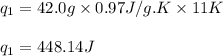
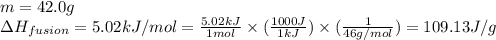
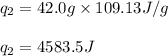
![m=42.0g\\c_l=2.3J/g.K\\T_2=78^oC\\T_1=-144^oC\\\Delta T=[T_2-T_1]=[78-(-144)]^oC=222^oC=222K](/tpl/images/0516/7715/36530.png)

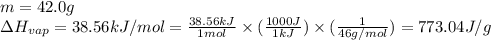
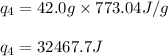
![[q_1+q_2+q_3+q_4]](/tpl/images/0516/7715/4ee42.png)
![[448.14+4583.5+21445.2+32467.7]J=58944.5J](/tpl/images/0516/7715/a8249.png)


