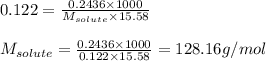Chemistry, 20.02.2020 03:33 cheerleader791
A. 0.2436 sample of an unknown substance was dissolved in 20.0mL of cyclohexane. The density of cyclohexane is 0.779 g/mL. The freezing point depression was 2.50 oC and the Kf value for cyclohecane is 20.5oC/m. Calculate the molality of the above solution and the molar mass of unknown.

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 17:30, tiffanyhmptn
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1
You know the right answer?
A. 0.2436 sample of an unknown substance was dissolved in 20.0mL of cyclohexane. The density of cycl...
Questions in other subjects:

Mathematics, 16.03.2020 03:00

Mathematics, 16.03.2020 03:00

English, 16.03.2020 03:01


Mathematics, 16.03.2020 03:01

Mathematics, 16.03.2020 03:01


Mathematics, 16.03.2020 03:01

Mathematics, 16.03.2020 03:01

Social Studies, 16.03.2020 03:01

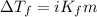
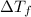 = depression in freezing point = 2.50°C
= depression in freezing point = 2.50°C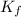 = molal freezing point elevation constant = 20.5°C/m
= molal freezing point elevation constant = 20.5°C/m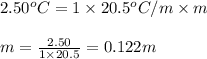
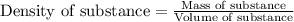
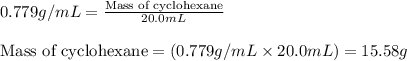
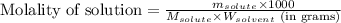
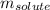 = Given mass of solute = 0.2436 g
= Given mass of solute = 0.2436 g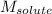 = Molar mass of solute = ? g/mol
= Molar mass of solute = ? g/mol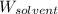 = Mass of solvent (cyclohexane) = 15.58 g
= Mass of solvent (cyclohexane) = 15.58 g