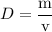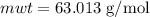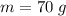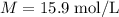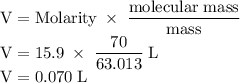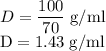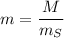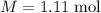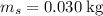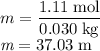Chemistry, 20.02.2020 03:38 anabelleacunamu
The concentration of commercially available concentrated nitric acid is 70.0 percent by mass, or 15.9 M. Calculate the density and molality of the solution.\

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, kimberlyrios12p0ts98
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2


Chemistry, 22.06.2019 10:10, andersonemma2222
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 11:00, 21villalobosjabez
Which type of fossil does this image depict?
Answers: 1
You know the right answer?
The concentration of commercially available concentrated nitric acid is 70.0 percent by mass, or 15....
Questions in other subjects:




Mathematics, 21.07.2019 23:00



History, 21.07.2019 23:00


Chemistry, 21.07.2019 23:00