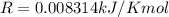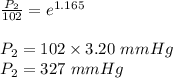Chemistry, 20.02.2020 01:51 olaffm9799
The vapor pressure of ethanol is 1.00 × 102 mmHg at 34.90°C. What is its vapor pressure at 60.21°C? (ΔHvap for ethanol is 39.3 kJ/mol.)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, kitt90335
Asample contains 16.75 g of the radioisotope u-236 and 50.25 g of its daughter isotope, th-232. how long did it take for decay to take place if one half-life of u-236 is 23 million years? 46 million years 69 million years 92 million years 115 million years
Answers: 3

Chemistry, 21.06.2019 18:30, tae8002001
How much energy moves onto the next level, in an energy pyramid
Answers: 1

Chemistry, 22.06.2019 10:00, halohero7
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

You know the right answer?
The vapor pressure of ethanol is 1.00 × 102 mmHg at 34.90°C. What is its vapor pressure at 60.21°C?...
Questions in other subjects:

Mathematics, 07.04.2021 23:30



Mathematics, 07.04.2021 23:30

Advanced Placement (AP), 07.04.2021 23:30

Mathematics, 07.04.2021 23:30

Mathematics, 07.04.2021 23:30


Mathematics, 07.04.2021 23:30

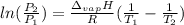
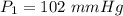

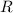 is the Universal Gas Constant.
is the Universal Gas Constant.