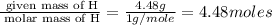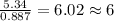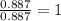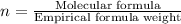Chemistry, 19.02.2020 23:18 zuleromanos
The reaction of equal molar amounts of benzene, C6H6, and chlorine, Cl2, carried out under special conditions, yields a gas and a clear liquid. Analysis of the liquid shows that it contains 64.03% carbon, 4.48% hydrogen, and 31.49% chlorine by mass and that is has a molar mass of 112.5 g/mol. The molecular formula will be determined. First, determine the number of moles of carbon in a 100 g sample.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:20, letsbestupidcx2314
Which of these conditions most likely produces an unstable isotope?
Answers: 1


Chemistry, 23.06.2019 04:20, vliu732
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1
You know the right answer?
The reaction of equal molar amounts of benzene, C6H6, and chlorine, Cl2, carried out under special c...
Questions in other subjects:

English, 18.10.2019 21:30



Mathematics, 18.10.2019 21:30






Mathematics, 18.10.2019 21:30