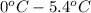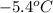Chemistry, 19.02.2020 22:48 moorega2100
Calculate the freezing point of a 2.9 m aqueous sucrose solution. (Assume that Kf for water is 1.86∘C/m.)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, bernicewhite156
Will give brainliest it is a lab from k12 here is the linkfor each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. type your answer here. (score for question 3: of 5 points) were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. type your answer here. (score for question 4: of 5 points) make a general statement about the reactivity of the metals in this experiment. type your answer here.
Answers: 2

Chemistry, 22.06.2019 03:30, jabper5522
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 06:00, Sarahinator04
0.09 moles of sodium sulfate in 12 ml of solution
Answers: 3
You know the right answer?
Calculate the freezing point of a 2.9 m aqueous sucrose solution. (Assume that Kf for water is 1.86∘...
Questions in other subjects:

Mathematics, 17.08.2021 03:50


Mathematics, 17.08.2021 03:50


Mathematics, 17.08.2021 03:50

History, 17.08.2021 03:50

Mathematics, 17.08.2021 03:50

Mathematics, 17.08.2021 03:50


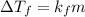
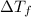 = depression in freeziing point
= depression in freeziing point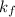 = molal deression in freezing point
= molal deression in freezing point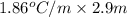
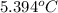
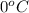 and the frreezing point of sucrose is calculated as follows.
and the frreezing point of sucrose is calculated as follows.