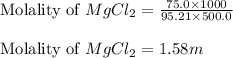Chemistry, 19.02.2020 21:00 mckenziet8930
Calculate the molality of 75.0 grams of MgCl2 (molar mass=95.21 g/mol) dissolved in 500.0 g of solvent.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, webbhlharryteach
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 03:40, 19thomasar
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 11:50, bellojamilet410
What substance has a mass of 9.5g and volume of 2.1cm^3
Answers: 2

Chemistry, 22.06.2019 12:30, hala201490
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1
You know the right answer?
Calculate the molality of 75.0 grams of MgCl2 (molar mass=95.21 g/mol) dissolved in 500.0 g of solve...
Questions in other subjects:


Spanish, 20.02.2021 20:30

Mathematics, 20.02.2021 20:30

Mathematics, 20.02.2021 20:30





Mathematics, 20.02.2021 20:30

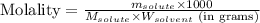
 = Given mass of solute (magnesium chloride) = 75.0
= Given mass of solute (magnesium chloride) = 75.0 = Molar mass of solute (magnesium chloride) = 95.21 g/mol
= Molar mass of solute (magnesium chloride) = 95.21 g/mol 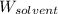 = Mass of solvent = 500.0 g
= Mass of solvent = 500.0 g