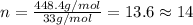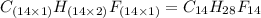Compound consists of carbon, hydrogen and fluorine. In one experiment, combustion of 2.50 g of the compound produced 3.926 g of CO2. Another sample weighing 5.00 g was found to contain 2.54 g of fluorine. The molar mass is found to be 448.4 g/mol. What are its empirical and molecular formulas? (AW(amu): C = 12.01, H = 1.008, F = 19.00)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, skaterwolf1317
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 13:00, netflixacc0107
Amixture with the same composition throughout is!
Answers: 1

Chemistry, 22.06.2019 22:40, destineysarah
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1

Chemistry, 22.06.2019 23:30, sweaversw9602
What are the similarities between compounds and mixtures?
Answers: 3
You know the right answer?
Compound consists of carbon, hydrogen and fluorine. In one experiment, combustion of 2.50 g of the c...
Questions in other subjects:

English, 18.08.2021 17:00

Mathematics, 18.08.2021 17:00


History, 18.08.2021 17:00

Mathematics, 18.08.2021 17:00

Mathematics, 18.08.2021 17:00


Chemistry, 18.08.2021 17:00


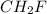 and
and 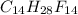 respectively
respectively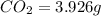
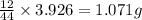 of carbon will be contained.
of carbon will be contained.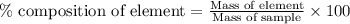 ......(1)
......(1)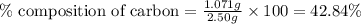
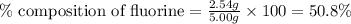
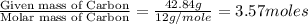
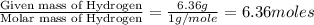
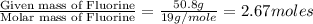
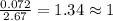
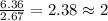
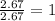
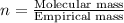
![12+(2\times 1)+19]=33g/mol](/tpl/images/0516/0009/ccdc5.png)