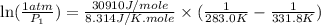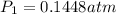Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, kiki197701
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3

Chemistry, 21.06.2019 22:30, 20alondra04
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1


Chemistry, 22.06.2019 22:50, kanerobertrosss2213
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3
You know the right answer?
The normal boiling point of bromine is 58.8°C, and its enthalpy of vaporization is 30.91 kJ/mol. Wha...
Questions in other subjects:


History, 24.06.2021 21:40







Mathematics, 24.06.2021 21:40

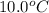 is 0.1448 atm.
is 0.1448 atm.
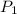 = vapor pressure of bromine at
= vapor pressure of bromine at 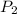 = vapor pressure of propane at normal boiling point = 1 atm
= vapor pressure of propane at normal boiling point = 1 atm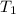 = temperature of propane =
= temperature of propane = 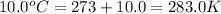
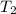 = normal boiling point of bromine =
= normal boiling point of bromine = 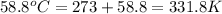
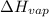 = heat of vaporization = 30.91 kJ/mole = 30910 J/mole
= heat of vaporization = 30.91 kJ/mole = 30910 J/mole