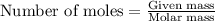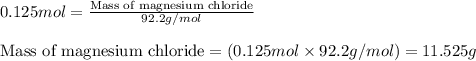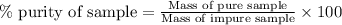Chemistry, 19.02.2020 05:58 jalenamaynard3989
A 12.00g sample of MgCl2 was dissolved in water. The solution was then treated with 0.2500mol of AgNO3 to precipitate all the chloride ions from the solution. Calculate the purity (as a mass percentage) of MgCl2 in the sample?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:30, Kathryn014
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1

You know the right answer?
A 12.00g sample of MgCl2 was dissolved in water. The solution was then treated with 0.2500mol of AgN...
Questions in other subjects:

Mathematics, 29.07.2020 21:01

Mathematics, 29.07.2020 21:01


Mathematics, 29.07.2020 21:01

Mathematics, 29.07.2020 21:01

Mathematics, 29.07.2020 21:01

English, 29.07.2020 21:01

Social Studies, 29.07.2020 21:01


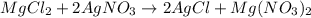
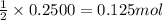 of magnesium chloride
of magnesium chloride