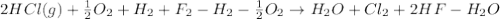Chemistry, 19.02.2020 02:23 Javanese5987
From the following enthalpies of reaction,
4 HCl (g) + O2 (g) → 2 H2O (l) + 2 Cl2 (g) ∆H = -202.4 kJ/mol 1/2 H2 (g) + ½ F2 (g) → HF (l) ∆H = -600.0 kJ/mol H2 (g) + ½ O2 (g) → H2O (l) ∆H = -285.8 kJ/mol
Calculate ∆Hrxn for 2 HCl (g) + F2 (g) → 2 HF (l) + Cl2 (g) Just input a number.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:00, Powerhickory1313
Which of the following statements is true? question 4 options: nuclear decay rates vary with the conditions of the reaction, but chemical reaction rates do not. chemical reaction rates vary with the conditions of the reaction, but nuclear decay rates do not. neither chemical reaction rates nor nuclear decay rates vary with the conditions of the reaction. both chemical reaction rates and nuclear decay rates vary with the conditions of the reaction.
Answers: 1

Chemistry, 22.06.2019 02:00, lydiadmanautou04
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 09:00, lrasanaoaksandfurana
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1

Chemistry, 22.06.2019 10:00, sdlesley66
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1
You know the right answer?
From the following enthalpies of reaction,
4 HCl (g) + O2 (g) → 2 H2O (l) + 2 Cl2 (g) ∆H = -2...
4 HCl (g) + O2 (g) → 2 H2O (l) + 2 Cl2 (g) ∆H = -2...
Questions in other subjects:

History, 18.07.2019 00:00



Mathematics, 18.07.2019 00:00


Mathematics, 18.07.2019 00:00

Chemistry, 18.07.2019 00:00

Mathematics, 18.07.2019 00:00

Computers and Technology, 18.07.2019 00:00

Biology, 18.07.2019 00:00

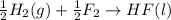 ΔH=-600.0 KJ/mol
ΔH=-600.0 KJ/mol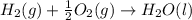 ΔH= -285.8 KJ/mol
ΔH= -285.8 KJ/mol