Given the following at 25°C, calculate for HCN(g) (in kJ/mol) at 25°C.
; ΔH= –870.8 kJ
...


Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:50, nnaomii
What is the composition, in atom percent, of an alloy that consists of 4.5 wt% pb and 95.5 wt% sn? the atomic weights for pb and sn are 207.19 g/mol and 118.71 g/mol, respectively.(a) 2.6 at% pb and 97.4 at% sn(b) 7.6 at% pb and 92.4 at% sn(c)97.4 at% pb and 2.6 at% sn(d) 92.4 at% pb and 7.6 at% sn
Answers: 2

Chemistry, 21.06.2019 21:00, Gghbhgy4809
What pressure will be exerted by 0.675 moles of a gas at 25*c if it is in a 0.750-l container?
Answers: 1


Chemistry, 22.06.2019 00:30, portedon8644
13. calculate the initial concentration (before precipitation) of carbonate ions after the addition of each 0.05 ml of solution b to the 1.00 l beaker of solution a. divide the work among group members and write the answers in the table in model 3. assume the volume change as solution b is added is negligible. 14. notice the initial concentrations of zn2+ - and cu2+ in the table in model 3. a. explain how these were obtained from the data in model 2. b. as solution b is added and precipitates form, do these initial concentrations change? 15. use the data in model 2 to indicate the presence of precipitate (either znco3 or cuco3) after each 0.05 ml addition of solution b in model 3. 16. use the initial concentrations of carbonate ions and zinc ions to calculate the reaction quotient, qsp for the zinc carbonate scenarios in model 3. divide the work among group members and write the answers in the table in model 3. 17. use the initial concentrations of carbonate ion and copper(ii) ions to calculate the qsp for the copper(ii) carbonate scenarios in model 3. divide the work among group members and write the answers in the table in model 3.
Answers: 3
You know the right answer?
Questions in other subjects:

Mathematics, 17.09.2020 02:01

Mathematics, 17.09.2020 02:01

Mathematics, 17.09.2020 02:01

Mathematics, 17.09.2020 02:01

Biology, 17.09.2020 02:01

Biology, 17.09.2020 02:01

Geography, 17.09.2020 02:01

Mathematics, 17.09.2020 02:01

English, 17.09.2020 02:01

Mathematics, 17.09.2020 02:01

![\Delta H^o_{rxn}=\sum [n\times \Delta H_f_{(product)}]-\sum [n\times \Delta H_f_{(reactant)}]](/tpl/images/0514/9325/e893d.png)
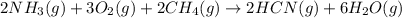
![\Delta H_{rxn}=[(2\times \Delta H_f_{(HCN(g))})+(6\times \Delta H_f_{(H_2O(g))})]-[(2\times \Delta H_f_{(NH_3(g))})+(3\times \Delta H_f_{(O_2(g))})+(2\times \Delta H_f_{(CH_4(g))})]](/tpl/images/0514/9325/ad92a.png)
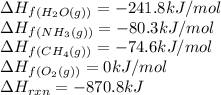
![-870.8=[(2\times \Delta H_f_{(HCN(g))})+(6\times (-241.8))]-[(2\times (-80.3))+(3\times (0))+(2\times (-74.6))]\\\\\Delta H_f_{(HCN(g))}=135.1kJ/mol](/tpl/images/0514/9325/0b580.png)


