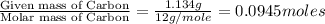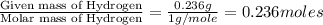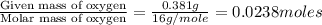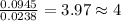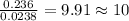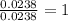Chemistry, 19.02.2020 00:00 anitadefrances
The compound known as diethyl ether, commonly referred to as ether, contains carbon, hydrogen, and oxygen. A 1.751 g sample of ether was combusted in an oxygen rich environment to produce 4.159 g of CO 2 ( g ) and 2.128 g of H 2 O ( g ) .
Insert subscripts to complete the empirical formula of ether.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:30, ethanmel21
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2


You know the right answer?
The compound known as diethyl ether, commonly referred to as ether, contains carbon, hydrogen, and o...
Questions in other subjects:

Biology, 08.09.2019 01:10





Mathematics, 08.09.2019 01:10


English, 08.09.2019 01:10


Mathematics, 08.09.2019 01:10

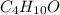
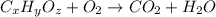
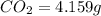
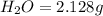
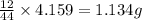 of carbon will be contained.
of carbon will be contained.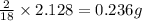 of hydrogen will be contained.
of hydrogen will be contained.