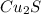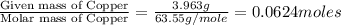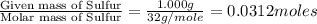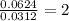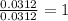Chemistry, 18.02.2020 23:54 Scoopaloop
In a chemical reaction, sulfur (S8) combines with copper to give a pure compound. If you start with 1.000 g of sulfur (S8) and obtain 4.963 g of pure compound. What is the empirical formula of this compound?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, mamabates181981
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1


Chemistry, 22.06.2019 18:00, jessicannoh5965
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1

Chemistry, 23.06.2019 06:30, madelineb6243
Which of these describes how heat is transferred by convection* a. sunlight travels through space without the aid of fluids or solids. b. warm air rises and takes the heat with it, eventually, it cools and sinks c. air at the equator rises and sinks at the poles. d. air molecules touch the warm ground, heating them up *not conduction
Answers: 3
You know the right answer?
In a chemical reaction, sulfur (S8) combines with copper to give a pure compound. If you start with...
Questions in other subjects:




Advanced Placement (AP), 05.05.2021 01:00

Social Studies, 05.05.2021 01:00



Mathematics, 05.05.2021 01:00

Spanish, 05.05.2021 01:00

Geography, 05.05.2021 01:00