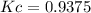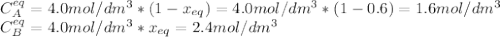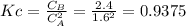The elementary reversible reaction
2A <-->B
is carried out in a flow re...

Chemistry, 18.02.2020 20:16 crispingolfer7082
The elementary reversible reaction
2A <-->B
is carried out in a flow reactor where pure A is fed at a concentration of 4.0 mol/dm 3 . If the equilib-rium conversion is found to be 60%,
(a) What is the equilibrium constant, KC if the reaction is a gas phase reaction?
(b) What is the KC if the reaction is a liquid-phase reaction?
(c) Write - rA solely as a function of conversion (i. e., evaluating all symbols) when the reaction isan elementary, reversible, gas-phase, isothermal reaction with no pressure drop with kA = 2 dm6 /mol•s and KC = 0.5 all in proper units.
(d) Repeat (c) for a constant-volume batch reactor.

Answers: 2


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 14:30, belindajolete
How do temperature and salinity affect deepwater currents? as temperatures and salinity levels of water increase, the water rises to the surface where it creates currents as it moves to colder regions. they create changes in wind direction, moving denser water in the same direction as the wind and causing the deepwater circulation patterns found in the ocean. they equalize the forces on undersea currents caused by the coriolis effect as they replace more dense water with less dense water. they create density differences that cause dense deepwater currents to flow toward the equator where they displace less dense, warmer water above them.
Answers: 2
You know the right answer?
Questions in other subjects:

History, 25.03.2021 19:50

Physics, 25.03.2021 19:50

Mathematics, 25.03.2021 19:50