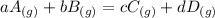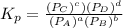Chemistry, 18.02.2020 19:34 iliketurtures
The equilibrium pressures below were observed at a certain temperature for the following reaction.
PNH₃ = 3.1 ✕ 10⁻² atm
PN₂ = 8.5 ✕ 10⁻¹ atm
PH₂ = 3.1 ✕ 10⁻³ atm
Calculate the value for the equilibrium constant at this temperature.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, rscott2649
In numbering carbon atoms in the parent chain of a hydrocarbon, why would you number from right to left, rather than left to right
Answers: 1

Chemistry, 22.06.2019 05:30, maddyjones4172
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3


Chemistry, 22.06.2019 20:00, montimcdaniel
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1
You know the right answer?
The equilibrium pressures below were observed at a certain temperature for the following reaction. <...
Questions in other subjects:

English, 25.08.2019 07:50



Computers and Technology, 25.08.2019 07:50

English, 25.08.2019 07:50


Biology, 25.08.2019 07:50

Social Studies, 25.08.2019 07:50


History, 25.08.2019 07:50