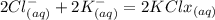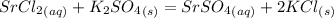When an aqueous solution of strontium chloride is added to an aqueous solution of potassium sulfate, a precipitation reaction occurs. Write the balanced net ionic equation of the reaction. Include charges on the ions, where applicable. Include coefficients only when they are different than ?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, nana54muller
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 23:00, ceejay8005
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1

Chemistry, 23.06.2019 01:00, akluke6059
Which statement characterizes synthetic polymers? a. they come from animals and plants. b. they are found in nature. c. they are made in a lab. d. they are components of starch.
Answers: 1
You know the right answer?
When an aqueous solution of strontium chloride is added to an aqueous solution of potassium sulfate,...
Questions in other subjects:






Chemistry, 08.12.2020 23:40


Mathematics, 08.12.2020 23:40

Mathematics, 08.12.2020 23:40

Health, 08.12.2020 23:40