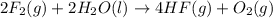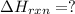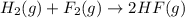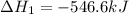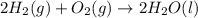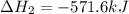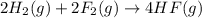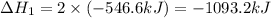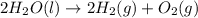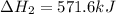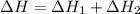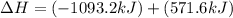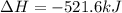Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, stelllllllllllllllla
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1


Chemistry, 22.06.2019 21:30, shiannethorn
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3
You know the right answer?
Given that H2 (g) + F2 (g) ⟶ 2HF (g) ΔH ∘ rxn = − 546.6 kJ 2H2 (g) + O2 (g) ⟶ 2H2O (l) ΔH∘rxn = − 57...
Questions in other subjects:


Social Studies, 18.02.2021 02:50

Chemistry, 18.02.2021 02:50




Mathematics, 18.02.2021 02:50


English, 18.02.2021 02:50

Chemistry, 18.02.2021 02:50

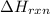 for the reaction is, -521.6 kJ
for the reaction is, -521.6 kJ