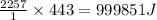Chemistry, 18.02.2020 06:27 rickyortega72701
As an athlete exercises, sweat is produced and evaporated to help maintain a proper body temperature. On average, an athlete loses approximately 443 g of sweat during an hour of exercise. How much energy is needed to evaporate the sweat that is produced? The heat of vaporization for water is 2257 J/g.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 22:30, robertss403
How many moles of kci are produced from 2.50 moles k
Answers: 1

Chemistry, 23.06.2019 07:00, MathChic68
Why do the strengths of london (dispersion) forces generally increase with increasing molecular size? choose one: a. heavier atoms have stronger attractions for each other than lighter atoms. b. dispersion forces are all equal in magnitude; there is no size dependence. c. dispersion forces arise from the attraction between the nuclei of atoms, and larger molecules have larger nuclei. d. dispersion forces arise from dipoles caused by the electron distribution being distorted. larger molecules have more electrons and, therefore, more distortions and a bigger force. e. dispersion forces depend on distance. larger molecules are farther apart and so the forces are smaller.
Answers: 2

Chemistry, 23.06.2019 10:30, cjtambasco
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 3.75 mol fe and 8.70 mol nio(oh) react?
Answers: 1
You know the right answer?
As an athlete exercises, sweat is produced and evaporated to help maintain a proper body temperature...
Questions in other subjects:

Social Studies, 31.03.2021 21:50

History, 31.03.2021 21:50

Mathematics, 31.03.2021 21:50

Business, 31.03.2021 21:50



Mathematics, 31.03.2021 21:50


Mathematics, 31.03.2021 21:50