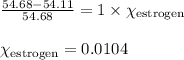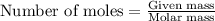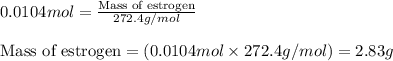Chemistry, 18.02.2020 05:04 taylorannsalazar
The vapor pressure of ethanol is 54.68 mm Hg at 25°C. How many grams of estrogen (estradiol), C18H24O2, a nonvolatile, nonelectrolyte (MW = 272.4 g/mol), must be added to 239.0 grams of ethanol to reduce the vapor pressure to 54.11 mm Hg ?

Answers: 3


Other questions on the subject: Chemistry




Chemistry, 23.06.2019 02:30, ggpro4life3000
Ascientist wants to know how individual lions within a pride interact with each other in their own environment. to do this, the scientist sedates and tags all of the lions within a pride. then, he places several remotely-controlled video cameras near the lions' den and performs an observational field study. he collects continuous video footage over the span of one year, analyzes the video, and then forms conclusions based on his observations.
Answers: 2
You know the right answer?
The vapor pressure of ethanol is 54.68 mm Hg at 25°C. How many grams of estrogen (estradiol), C18H24...
Questions in other subjects:

Social Studies, 04.02.2020 10:57





Biology, 04.02.2020 10:57

Social Studies, 04.02.2020 10:57



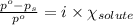
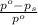 = relative lowering in vapor pressure
= relative lowering in vapor pressure 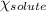 = mole fraction of solute = ?
= mole fraction of solute = ?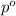 = vapor pressure of pure ethanol = 54.68 mmHg
= vapor pressure of pure ethanol = 54.68 mmHg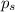 = vapor pressure of solution = 54.11 mmHg
= vapor pressure of solution = 54.11 mmHg