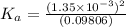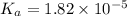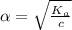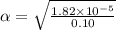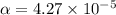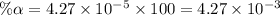Chemistry, 18.02.2020 05:22 Mangolinux7173
Start with 100.00 mL of 0.10 M acetic acid, CH3COOH. The solution has a pH of 2.87 at 25 oC. a) Calculate the Ka of acetic acid at 25 oC. b) Determine the percent dissociation for the solution.

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 13:00, netflixacc0107
Amixture with the same composition throughout is!
Answers: 1

Chemistry, 22.06.2019 21:00, lalaween098
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3
You know the right answer?
Start with 100.00 mL of 0.10 M acetic acid, CH3COOH. The solution has a pH of 2.87 at 25 oC. a) Calc...
Questions in other subjects:




Mathematics, 30.08.2021 05:30


Mathematics, 30.08.2021 05:30


English, 30.08.2021 05:30

Mathematics, 30.08.2021 05:30

Mathematics, 30.08.2021 05:30

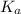 of acetic acid at
of acetic acid at 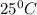 is
is 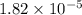
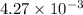
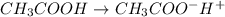
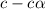
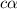
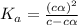
 = ?
= ?![pH=-log[H^+]](/tpl/images/0513/9443/15713.png)
![2.87=-log[H^+]](/tpl/images/0513/9443/3a07c.png)
![[H^+]=1.35\times 10^{-3}M](/tpl/images/0513/9443/01bcb.png)
![[CH_3COO^-]=1.35\times 10^{-3}M](/tpl/images/0513/9443/0a4ad.png)
![[CH_3COOH]=(0.10M-1.35\times 10^{-3}=0.09806M](/tpl/images/0513/9443/5420f.png)