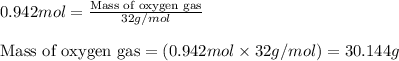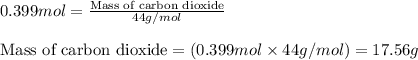Chemistry, 18.02.2020 04:19 jtbrown0093
A sample of 8.00 g of liquid 1‑propanol, C 3 H 8 O , is combusted with 49.3 g of oxygen gas. Carbon dioxide and water are the products. Write the balanced chemical equation for the reaction. Physical states are optional. chemical reaction: What is the limiting reactant? oxygen 1‑propanol How many grams of CO 2 are released in the reaction? mass of CO 2 : g How many grams of the excess reactant remain after the reaction is complete? mass of excess reactant remaining:

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:10, mpchop
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 02:40, hardwick744
Achange in the number of neutrons in an atom will change an blank . when the number of protons changes in an atom, a new element will form.
Answers: 2

Chemistry, 22.06.2019 06:10, andybiersack154
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 09:20, pandaman632
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1
You know the right answer?
A sample of 8.00 g of liquid 1‑propanol, C 3 H 8 O , is combusted with 49.3 g of oxygen gas. Carbon...
Questions in other subjects:

English, 10.04.2021 01:00

Mathematics, 10.04.2021 01:00

Physics, 10.04.2021 01:00



Mathematics, 10.04.2021 01:00




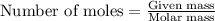 .....(1)
.....(1)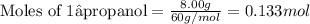
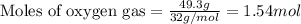
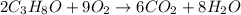
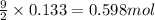 of oxygen gas
of oxygen gas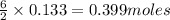 of carbon dioxide.
of carbon dioxide.