

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:40, cheesecake1919
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3


Chemistry, 23.06.2019 01:20, michellectucker1982
Use the de broglie's wave equation to find the wavelength of an electron moving at 7.3 × 106 m/s. show your work. note: h = plank's constant (6.62607 x 10-34 j s)
Answers: 1
You know the right answer?
Consider the following multistep reaction:
C+D⇌CD(fast)
CD+D→CD2(slow)
CD...
C+D⇌CD(fast)
CD+D→CD2(slow)
CD...
Questions in other subjects:

Mathematics, 06.01.2020 06:31

Mathematics, 06.01.2020 06:31



History, 06.01.2020 06:31

Mathematics, 06.01.2020 06:31

Mathematics, 06.01.2020 06:31


Business, 06.01.2020 06:31

English, 06.01.2020 06:31

![\text{Rate}=k'[C][D]^2](/tpl/images/0513/7830/4eb88.png)
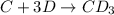
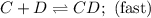
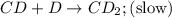
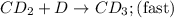
![\text{Rate}=k[CD][D]](/tpl/images/0513/7830/52b27.png) ......(1)
......(1)![K=\frac{[CD]}{[C][D]}](/tpl/images/0513/7830/c2f5d.png)
![[CD]=K[C][D]](/tpl/images/0513/7830/b2574.png)
![\text{Rate}=k.K[C][D]^2\\\\\text{Rate}=k'[C][D]^2](/tpl/images/0513/7830/6c083.png)


