
Chemistry, 18.02.2020 02:29 anggelkevin100
At a certain temperature this reaction follows second-order kinetics with a rate constant of 14.1 M-1s-1 : --->2SO3g+2SO2gO2g Suppose a vessel contains SO3 at a concentration of 1.44M . Calculate the concentration of SO3 in the vessel 0.240 seconds later. You may assume no other reaction is important.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, amandasantiago2001
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3


Chemistry, 23.06.2019 03:30, rniadsharri16
Select the correct lewis structure for fluorine which is group 7a element?
Answers: 1

Chemistry, 23.06.2019 04:00, hailey200127
What is the volume of 2.5 moles of nitrogen gas (n2)at standard temperature and pressure (stp)?
Answers: 1
You know the right answer?
At a certain temperature this reaction follows second-order kinetics with a rate constant of 14.1 M-...
Questions in other subjects:




Mathematics, 22.08.2020 19:01

Physics, 22.08.2020 19:01





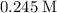 . (Moles-per-liter.)
. (Moles-per-liter.) 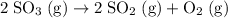 .
.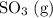 is the only reactant in this reaction. Only the concentration of
is the only reactant in this reaction. Only the concentration of ![x = \left[\rm SO_3\; (g)\right]](/tpl/images/0513/7419/fbcd2.png) (that's the concentration of
(that's the concentration of  (with respect to time
(with respect to time 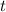 ) is proportional to
) is proportional to 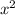 . In other words,
. In other words,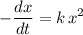 ,
,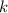 is the rate constant of the reaction. Note the negative sign in front of the fraction. Reactants are consumed in a reaction, so their concentrations would become smaller.
is the rate constant of the reaction. Note the negative sign in front of the fraction. Reactants are consumed in a reaction, so their concentrations would become smaller.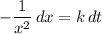 .
.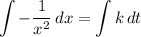 .
.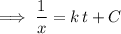 .
.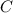 here is fixed; its exact value depends on the initial concentration of the reaction. Rearrange to obtain an equation for
here is fixed; its exact value depends on the initial concentration of the reaction. Rearrange to obtain an equation for 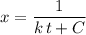 .
.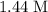 at the beginning of the reaction. As a result,
at the beginning of the reaction. As a result, 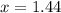 at
at 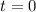 .
.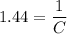 .
.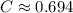 .
.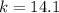 . Calculate the value of
. Calculate the value of 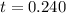 :
: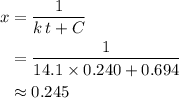 .
.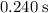 .
.

