

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:50, amandamac7339
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 23.06.2019 06:40, hackman1216
The combustion of methane, ch4, releases 890.4kj/mol. that is, when one mole of methane is burned,890.4 kj are given off to the surroundings. this meansthat the products have 890.4 kj less than the reactants. thus, ah for the reaction = - 890.4 kj. a negative symbolforah indicates an exothermic reaction. ch (g) + 20 (g)> co2 (g) + 2 h0 (1); ah = - 890.4 kga) how much energy is given off when 2.00 mol of ch, are burned? b) how much energy is released when 22.4g of ch. areburned?
Answers: 1

Chemistry, 23.06.2019 10:30, erikloza12pdidtx
Which of the following pairs of elements is most likely to form an ionic compound? a oxygen and fluorine b sodium and aluminum c calcium and chlorine d nitrogen and sulfur
Answers: 1
You know the right answer?
In a reaction, gaseous reactants form a liquid product. The heat absorbed by the surroundings is 1.1...
Questions in other subjects:




Mathematics, 06.06.2021 01:30

Mathematics, 06.06.2021 01:30



Health, 06.06.2021 01:30


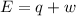


 is 1155 kJ
is 1155 kJ
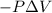 {Work done on the system is positive as the final volume is lesser than initial volume}
{Work done on the system is positive as the final volume is lesser than initial volume}
 (1kcal=4.184 kJ)
(1kcal=4.184 kJ)


